To respect quality standards and protect consumer health, pharmaceutical products must be tested to identify any defects in the packaging seal and structural integrity.
Container Closure Integrity Testing (CCIT) identifies a wide range of packaging defects, rejecting products that do not comply with standards. This gives the manufacturer the certainty that the products being placed in the market have been inspected and that they will withstand storage and transportation.
Below, we compare two widely used CCIT techniques: Vacuum Decay and High Voltage Leak Detection (HVLD), highlighting when and why one may be preferred over the other.
VDM
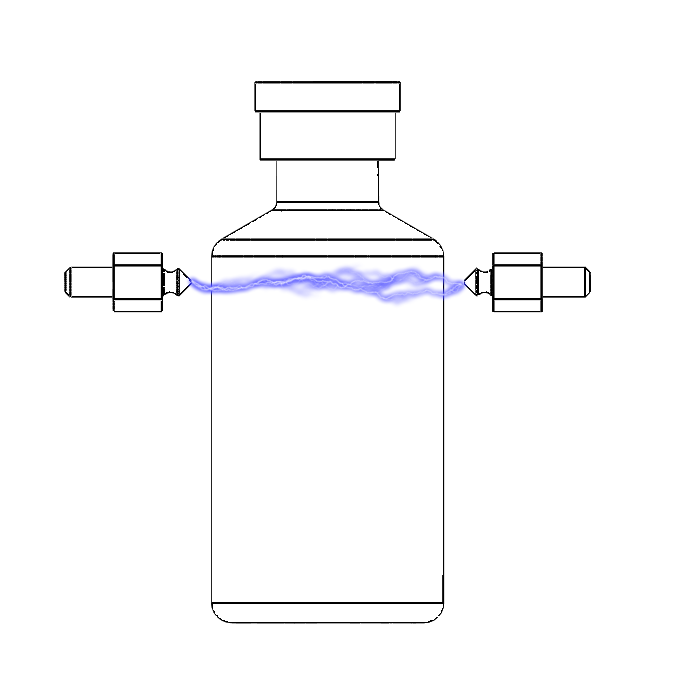
HVLD
High Voltage Leak Detection and Vacuum Decay: an overview
Choosing the right container closure integrity testing (CCIT) method can impact product safety, regulatory compliance, and operational efficiency.
What Is HVLD?
High Voltage Leak Detection detects holes or cracks using electricity to check the integrity of containers made of an insulating material (such as glass) containing products that conduct electricity (such as a drug in a liquid solution). In this test, a potential difference is applied to the container using electrodes, and conductivity variations are measured. If packaging integrity is compromised, the machine will detect a change in conductivity, indicating non-compliance with the expected quality standard.
What are HLVD Strengths and Differences?
The main strength of the High Voltage Leak Detection or HVLD is its ability to detect very small holes while operating at high speeds.
However, this technique also has limitations:
- Package Material and Contents Can Influence Results. Both the material from which the container is made, and its content influences the test result, since the result depends on the conductivity of the medium. This means that if the packaging is modified significantly, the machine will have to be set up again to guarantee reliable results.
- Package Shape Can Include Results. In addition to the package material, its shape can also influence how an HVLD test is conducted. In some cases, it is not possible to inspect the entire container in a single step so several tests are needed to check the tightness of different sections of the container, and the entire package cannot always be inspected.
- Metal Parts Cannot Be Tested. Packaging must be made of an insulating material for testing to be able to use this technology. If containers with metal parts (e.g., vials with an aluminium cap) are tested, the metal section cannot be inspected.
What Type of Pharmaceutical Containers are Best for HVLD?
HVLD is best suited for rigid, liquid-filled pharmaceutical containers with conductive solutions and insulating walls.
- Glass and plastic pre-filled syringes (with conductive liquid content)
- Glass vials containing saline or buffered solutions
- Ampoules filled with liquid injectables
- Cartridges used in auto-injector pens (if solution is conductive)
- Sealed rigid containers where full external inspection is possible
- Applications where speed and high throughput are required
- Ideal for sterile fill-finish lines using aqueous solutions
- Regulated production environments using narrow packaging formats
Limitations: Not suitable for flexible packaging, metal components (e.g., aluminum caps), or non-conductive liquids like oils, suspensions, or viscous biologics.
What is Vacuum Decay Leak Detection?
The Vacuum Decay Method detects pressure changes that can occur when a closed and sealed product is exposed to a vacuum. The products to be checked are placed in test chambers in which a vacuum is applied. Then, pressure values are read twice, a few seconds apart. If the difference between the two pressure values exceeds a threshold established during machine set-up, the container has a leak and must be rejected.
Vacuum Decay Method strengths:
- Can Inspect Material of any Shape or Size. Vacuum Decay inspection technology can inspect a product completely regardless of its shape, material, content, or presence of electrically conductive parts. The VDM technology can provide reliable seal results for a much wider range of containers than HVLD solutions.
- Can Inspect Any Material. Vacuum decay testing machines can be used for products of any shape and made of any non-porous material. The packaging can contain products of any kind: liquids, solids, powders, freeze-dried products or in a vacuum.
The main drawback of VDM is that the inspection speed is lower than HVLD. Although currently, machines with VDM technology can test up to 600 products per minute, as is its sensitivity, the VDM can detect holes measuring 1 micron in laboratory applications, and 5-10 microns in in-line applications.
What Type of Pharmaceutical Containers are Best for Vacuum Decay Testing?
VDM offers deterministic, non-destructive leak detection for a broad range of container formats and materials.
- IV bags, including multi-chamber and non-conductive formulations
- Flexible pouches (e.g., parenteral nutrition, infusion therapy)
- Blister packs for tablets or capsules
- Plastic vials, bottles, and containers of any shape
- Lyophilized (freeze-dried) product containers
- Powder-filled or vacuum-sealed containers
- Products with aluminum seals or metallic closures
- Large volume containers (1L+), including plasma derivatives
- Non-conductive or viscous liquids, like suspensions or emulsions
- Lab environments or small-batch production, requiring high sensitivity
- R&D, stability, and QA testing scenarios
Bonus: Vacuum Decay is the preferred method for USP <1207>, EU Annex 1, and ASTM F2338 compliance.
High Voltage Leak Detection vs. Vacuum Decay (Comparison Chart)
|
Category |
High Voltage Leak Detection (HVLD) |
Vacuum Decay (VD) |
|
Test Method |
Detects conductivity when a leak allows microcurrent to pass through a defect |
Measures pressure changes in a vacuum chamber due to gas leakage |
|
Container Compatibility |
Best for rigid, conductive liquid-filled containers like pre-filled syringes and glass vials |
Ideal for flexible and rigid packaging, including IV bags, pouches, blisters, and non-conductive fluids |
|
Leak Detection Sensitivity |
Limited sensitivity, especially for non-conductive or viscous solutions |
High sensitivity to microleaks, even with plasma or non-conductive fluids |
|
Impact of Solution Conductivity |
Requires conductive solutions for accurate results |
Not dependent on liquid conductivity |
|
Speed |
Fast for small rigid formats |
Moderate, but consistent and scalable for lab or low-volume production |
|
Ideal Use Cases |
Glass ampoules, pre-filled syringes, sealed rigid vials |
IV bags, pouches, blister packs, large flexible containers |
Why Choose the Vacuum Decay Method?
Bonfiglioli Engineering has adopted the VDM for its versatility and possibility to create machines tailored to diverse types of packaging containers and their contents.
In its 50-year history of manufacturing and installing leak detection machines, Bonfiglioli Engineering has delivered over 5000 machines with the VDM technology to customers worldwide. This experience allows Bonfiglioli Engineering to offer its customers VDM machines for any application, whether for use in the laboratory or in line, covering a wide range of products and easily adapting to the customer's needs.
Questions? Reach out to our team of experts at any time.
Take a look at our IVB Flex vacuum decay inspection machine in action below.
FAQs
HVLD is a probabilistic leak test method that relies on detecting electrical conductivity in liquid-filled containers. Vacuum Decay, on the other hand, is a deterministic method which detects leaks by monitoring pressure changes in a vacuum chamber.
Vacuum Decay Method (VDM) is widely recognized as a regulatory-compliant leak detection method for pharmaceutical applications. It is a deterministic, non-destructive technique and is explicitly referenced in key global standards and guidelines, including:
- USP <1207> – U.S. Pharmacopeia standard for container closure integrity testing (CCIT)
- ASTM F2338 – Standard test method for VDM-based leak detection
- EU GMP Annex 1 – European guidance for the manufacture of sterile medicinal products
- 21 CFR Part 11 – Compliance possible when paired with digital audit trails and electronic records
Because of its deterministic nature and quantitative results, Vacuum Decay aligns with current regulatory expectations for CCIT and supports inspection readiness during audits.
In contrast, High Voltage Leak Detection is considered a probabilistic method and is not explicitly listed in USP <1207> or EU Annex 1 as a preferred or referenced technique. While it is still used in some applications, especially with rigid containers like glass vials or syringes containing conductive solutions, it may not fulfill the latest regulatory preference for deterministic methods.
Both HVLD and Vacuum Decay can experience false rejects if surface moisture interferes with test signals. However, modern Vacuum Decay systems use AI-driven algorithms to compensate for humidity variability, delivering more accurate results and significantly reducing false positives caused by wet surfaces.

